Practitioner Portal > Doing Research
“Instead of just mooching through the research literature, consciously or unconsciously picking out papers here and there that support [our] pre-existing beliefs [we] take a scientific, systematic approach to the very process of looking for scientific evidence, ensuring that [our] evidence is as complete and representative as possible of all the research that has ever been done.” (Ben Goldacre 2012 Bad Pharma: How drug companies mislead doctors and harm patients, London: Fourth Estate)
If you are based in Ireland and have done a piece of research that may be of interest to others working in the drugs area you can submit it to our collection by sending us an email at drugslibrary@hrb.ie. Please get in touch if you have any questions.
• Paper 1 How to apply for research funding p.3
• Paper 2 How to match research designs to organisational issues in health & social care p.16
• Paper 3 Critical analysis of research literature p.31
• Paper 4 How to conduct a literature review p.44
• Paper 5 How to design quantitative research in applied settings p.57
• Paper 6 How to engage with stakeholders through qualitative research p.68
• Paper 7 How to conduct mixed methods research p.87
• Paper 8 How to conduct action research in healthcare settings p.97
• Paper 9 Research ethics: guidelines for practice p.106
• Paper 10 How to analyse quantitative data p.112
• Paper 11 How to analyse qualitative data p.120
European Union Drugs Agency web-resource: Best practice portal (external website)
The K2A cycle is made up of 4 stages, with continuous user engagement throughout all stages of the cycle. 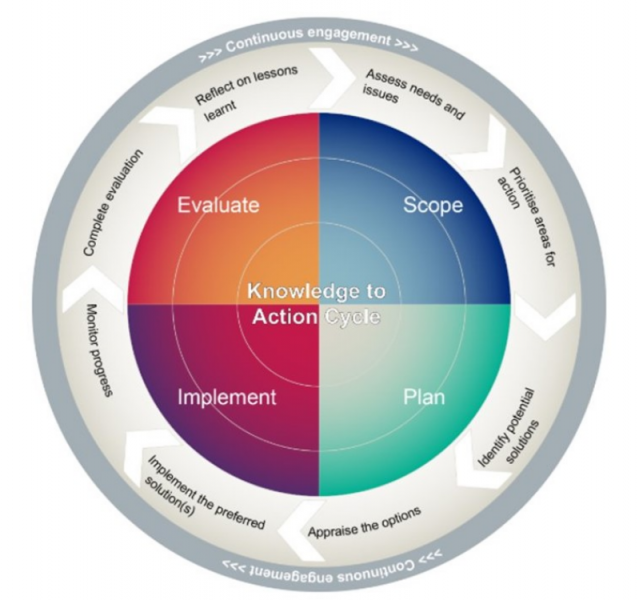
Stage 1: Scope - Assess user needs, and think about the problems you are trying to solve with your work. Prioritise the subjects you will need to address.
Stage 2: Plan - Identify some possible solutions to the problems you are trying to solve. Review all the different solutions and decide on the best ones to use.
Stage 3: Implement - Use the best solutions to solve problems for your users. Monitor whether the solutions are effective as the project progresses.
Step 4: Evaluate - Evaluate your work. Think about the lessons you have learned from the project.
• Learn about evidence-informed decision-making, and why research is an essential element of it.
• Understand the different scenarios when using evidence can help you, as well as the types of evidence you might need at different stages of development.
• Explore different types of evidence, how to choose the most appropriate and how to judge its quality.
• Get advice on finding the right evidence to support your case, and how to get your message across once you have it.
Several reports provide guidance on the use of stigmatising language:
- (2018) Stop the stigma campaign. Dublin: Citywide.
- (2021) Edging forward: how the UN’s language on drugs has advanced since 1990 (version 2). International Drug Policy Consortium.
- (2020) Words matter - terms to use and avoid when talking about addiction. NIDAMED.
- (2022) Reporting of substance media toolkit. Scottish Families Affected by Alcohol and Drugs, Adfam.
- (2020) Moving beyond 'people first' language: a glossary of contested terms in substance use. Scottish Drugs Forum.
- (2020) Words matter! Language statement & reference guide. INPUD Secretariat.
For other publications in this area, click for research related to stigma or click for research related to language.
E-learning for healthcare by NHS, Health Education England
‘Building the Foundations’ includes three modules to enable researchers to assess their current level of skill in literature searching, find out more about resources and get started planning a search.
- Module 1 Introduction to searching
- Module 2 Where do I start searching?
- Module 3 How do I start to develop a search strategy?
- Module 4 Too many results? How to narrow your search
- Module 5 Too few results? How to broaden your search
- Module 6 Searching with subject headings
Asking the right question [2.54 minutes]
Finding the evidence [2.20 minutes]
Assessing the evidence [2.38 minutes]
Synthesising the evidence [3.15 minutes]
Plan your search strategy by framing your question.
Use PICO to break down your topic into separate ‘concepts’ to research interventions.
• Population / Patient
• Intervention
• Comparison / Counter Intervention
• Outcome
CASP - PICO search strategy tips & examples
Youtube Finding the evidence 1 - Using PICO to formulate a search question
Youtube Finding the evidence 2 - Turning search terms into a search strategy
Youtube Finding the evidence 3 - Turning your search strategy into results: PubMed demonstration
PICO is often associated with clinical-type questions, so other tools may be useful:
SPIDER – Sample, Phenomenon of Interest, Design, Evaluation, Research Type
SPICE – Setting. Perspective. Intervention or Exposure or Interest, Comparison, Evaluation[For more details on these (external) tools click here]
• WHO - ECDD information repository
• Cochrane Library
• Drug and Alcohol Findings (archive)
• European Union Drugs Agency (EUDA)
• National Institute for Health and Care Excellence (NICE)
• Public Health Scotland - substance use
Other free health databases include
• Lenus: the Irish health repository contains HSE publications and the freely accessible collected output of more than 130 Irish health organisations.
• Pubmed has more than 27 million citations for biomedical literature. Where it is available, records include links to full-text content from PubMed Central and publisher web sites
• BMJ Best Practice provides healthcare professionals with quick, easy access to the latest information and evidence for diagnosis and treatment decisions. Access is enabled via the Irish national IP. BMJ Best Practice can also be accessed anytime via the mobile responsive website or the app [requires free login for those in Ireland].
• Medline Plus present high-quality, relevant health and wellness information.
• Trip Database is a tool for you to find and use high-quality clinical research evidence.
Not all references in databases provide access to the full text of a publication. If you come across an article that you need for your research you may have access through your organisation’s library (such as the HSE library). If not please contact us to see if we can be of help obtaining the article.
Department of Health (2013) How to conduct a literature search. Dublin: Department of Health
Robert Calder (2021) What is a systematic review? A step-by-step guide for beginners. SSA Blog,
Literature review
In order to put your research into context it is important to briefly describe what others have written on your topic. A literature review surveys scholarly articles, books and other sources (e.g. dissertations, conference proceedings) relevant to a particular issue, area of research, or theory, providing a description, summary, and critical evaluation of each work. The purpose is to offer an overview of significant literature published on a topic.
A literature review requires several stages (from University of California, Santa Cruz)
• Problem formulation – which topic or field is being examined and what are its component issues?
• Literature search – finding materials relevant to the subject being explored
• Data evaluation – determining which literature makes a significant contribution to the understanding of the topic
• Analysis and interpretation – discussing the findings and conclusions of pertinent literature
Literature reviews should comprise the following elements:
• An overview of the subject, issue or theory under consideration, along with the objectives of the literature review
• Division of works under review into categories (e.g. those in support of a particular position, those against, and those offering alternative theses entirely)
• Explanation of how each work is similar to and how it varies from the others
• Conclusions as to which pieces are best considered in their argument, are most convincing of their opinions, and make the greatest contribution to the understanding and development of their area of research
In assessing each piece, consider:
• Provenance – What are the author's credentials? Are the author's arguments supported by evidence (e.g. primary historical material, case studies, narratives, statistics, recent scientific findings)?
• Objectivity – Is the author's perspective even-handed or prejudicial? Is contrary data considered or is certain pertinent information ignored to prove the author's point?
• Persuasiveness – Which of the author's theses are most/least convincing?
• Value – Are the author's arguments and conclusions convincing? Does the work ultimately contribute in any significant way to an understanding of the subject?
Identifying and evaluating types of research:
Critical appraisal is the process of carefully and systematically examining research to judge its trustworthiness, and its value and relevance in a particular context.
What is a Systematic Review? (from Critical Appraisal Skills Programme (CASP))
Frequently there will have been more than one study addressing a particular health question. In such circumstances it is logical to collect all these studies together and base conclusions on the cumulated results. However the same scientific principles as would be expected in the original studies need to be applied to the identification, sorting and analysis of potentially relevant studies. This is what is meant by a systematic review. The most obvious sign that a review is systematic will be the presence of a methods section. Meta-analysis is the statistical process of combining the results from several studies that is often part of a systematic review.
CASP Systematic Review Checklist
What is a Randomised Controlled Trial (RCT)?
An RCT is a type of interventional or experimental study design. Participants (individuals or groups) are randomly allocated to receive either the new intervention being tested or a control treatment (usually the standard treatment or a placebo). Each arm of the study is then followed up and the amount or severity of the disease measured in the intervention group and compared with the control group. RCTs are by definition prospective.
CASP Randomised Controlled Trial Checklist
What is Qualitative research?
A qualitative study examines the experiences and beliefs of people from their own perspective. It can take many forms including in-depth interviews and focus-groups with analysis attempting to identify underlying themes. Verbatim quotes of participants can be used to illustrate these themes. It is often used in social sciences, healthcare, and education. Qualitative research aims to explore complex issues and experiences through interviews, observations, and focus groups. It allows researchers to gather rich and descriptive information, giving voice to participants' experiences and perspectives.
CASP Qualitative Checklist
What is a Cross-sectional study?
A cross-sectional study serves as an observational tool, where researchers capture data from a cohort of participants at a singular point. This approach gives a 'snapshot'— a brief glimpse into the characteristics or outcomes prevalent within a designated population at that precise point in time. The primary aim here is not to track changes or developments over an extended period but to assess and quantify the current situation regarding specific variables or conditions. Such a methodology is instrumental in identifying patterns or correlations among various factors within the population, providing a basis for further, more detailed investigation.
CASP Cross-sectional Checklist
What is a Cohort study?
A cohort study, also known as a follow-up or longitudinal study, is another observational study design. In this study a population who do not have the health outcome or disease of interest are first divided into those who are exposed to a risk factor and those who are not. Alternatively exposed and unexposed populations may be chosen separately. Irrespective, both groups are then followed, often over long periods of time. At the end of the period of observation the incidence of disease or frequency of health outcome in the exposed group is compared to that in the unexposed group. The study is generally prospective as it looks forward from potential cause to consequence.
CASP Cohort Study Checklist
What is a Case-Control study?
A case-control study belongs to the observational group of studies. It begins by choosing individuals who have a health outcome or disease whose cause you want to investigate. These are the cases. Controls without the health outcome are then chosen. You then determine the proportion of cases who were exposed to any risk factor of interest in the past, and compare this with the proportion exposed in the control group. The study is generally retrospective because it looks backwards in time to the earlier exposures of individuals.
CASP Case Control Checklist
Other research checklists
• CASP Clinical Prediction Rule Checklist
• CASP Diagnostic Checklist
• CASP Economic Evaluation Checklist
Other critical appraisal resources:
Population health toolkit
Population health is an approach aimed at improving the health of an entire population. It is about improving the physical and mental health outcomes and wellbeing of people, whilst reducing health inequalities within and across a defined population. It includes action to reduce the occurrence of ill-health, including addressing wider determinants of health, and requires working with communities and partner agencies.
The CARS Checklist for Research Source Evaluation from the article: Evaluating Internet Research Sources by Robert Harris, 2020
Few information sources will meet every criterion in the list, and even those that do may not possess the highest level of quality possible. But if you learn to use the criteria in this list, you will be much more likely to separate the high quality information from the poor quality information.
• Credibility trustworthy source, author’s credentials, evidence of quality control, known or respected authority, organizational support.
Goal: an authoritative source, a source that supplies some good evidence that allows you to trust it.
• Accuracy up to date, factual, detailed, exact, comprehensive, audience and purpose reflect intentions of completeness and accuracy.
Goal: a source that is correct today (not yesterday), a source that gives the whole truth.
• Reasonableness fair, balanced, objective, reasoned, no conflict of interest, absence of fallacies or slanted tone.
Goal: a source that engages the subject thoughtfully and reasonably, concerned with the truth.
• Support listed sources, contact information, available corroboration, claims supported, documentation supplied.
Goal: a source that provides convincing evidence for the claims made, a source you can triangulate (find at least two other sources that support it).
This page was last updated in July 2025