Lynn, Ena (2020) Repeated cross-sectional study of factors associated with pregabalin-positive poisoning deaths in Ireland. Drugnet Ireland, Issue 72, Winter 2020, pp. 16-17.
| Preview | Title | Contact |
|---|---|---|
|
PDF (Drugnet Ireland)
600kB |
Introduction
Pregabalin is a prescribed medication licensed in Europe for use in the treatment of epilepsy, neuropathic pain, and generalised anxiety disorder.1 However, the pharmacokinetic properties of pregabalin, which include its rapid absorption, fast onset of its relaxant and sedative effects, and its reduced withdrawal symptoms, can lead to the potential risk of misuse. As outlined in a previous Drugnet Ireland article,2 in Ireland, the rates of prescribing pregabalin have increased in line with an increase in poisoning deaths, where pregabalin was present on toxicology.
The increasing use of pregabalin and its presence in poisoning deaths, particularly with opioids, highlight it as a potential drug of abuse. Misuse of pregabalin has been reported, especially among people with a history of opioid misuse,3,4 people in opioid substitution treatment,5 and people in prisons.6 A recent Irish study, using data from the National Drug-Related Deaths Index (NDRDI), examined factors associated with pregabalin-positive poisoning deaths (PPPD) between 2013 and 2016.7
Methods
Data for this study were extracted from the NDRDI. The NDRDI’s definition of a poisoning death is a death directly due to the toxic effect of one or more substances on the body. For this study, PPPD included all poisoning deaths where pregabalin was present on the toxicology report, with years of death 2013–2016 inclusive as the observation period. Analysis included univariate and multivariate logistic regression to estimate unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) for factors associated with PPPD (primary outcome) by logistic regression models for the total sample and stratified by gender.
Results
Pregabalin
Pregabalin was present on 240 (16%) toxicology reports of 1,489 poisoning deaths, significantly rising from 18 (5%) in 2013 to 94 (27%) in 2016. While the total number of poisoning deaths appeared to decrease over the reporting period, there was an increase in PPPD (see Table 1). Women, opioid misuse, being in receipt of treatment for problem drug use, and year of death (2016 vs 2013) were associated with increased odds of PPPD. Alcohol dependence was associated with reduced odds of PPPD. Analysis was then stratified by gender. For men, opioid misuse, being in receipt of treatment for problem drug use, and year of death were associated with increased odds of PPPD, while alcohol dependence was associated with reduced odds of PPPD. For women, being in receipt of treatment for problem drug use and year of death were associated with increased odds of PPPD.
Polydrugs
Polydrugs were present on the toxicology reports of all PPPD (n=240). Almost all (234, 97.5%) had a positive toxicology report for other central nervous system (CNS) depressant drugs, mainly opioids (211, 88%), followed by benzodiazepines (207, 86%) and alcohol (58, 24%). Methadone (122, 51%) was the main opioid reported in PPPD, followed by heroin (44, 18%). The odds of opioid drugs being present on toxicology reports (versus none) were 6.54 times more likely for PPPD than pregabalin-negative poisoning deaths (PNPD), with the odds for women twice that for men.
Two or more other CNS depressant drugs were present in the majority (205, 85%) of PPPD toxicology reports. The odds of two or more CNS depressant drugs being present on toxicology reports (versus none) were 10.38 times more likely for PPPD compared with PNPD, with the odds for women three times that for men. This is significant as pregabalin can exacerbate the side-effects of CNS depressant drugs, and with multiple CNS depressant drugs present in PPPD, the synergistic effect of the combination of these drugs increases the risk of death.
The odds of antidepressant drugs present on toxicology (versus none) were 5.49 times more likely for PPPD than PNPD; for antipsychotic drugs, the odds ratio was 3.82; and for Z-drugs it was 2.74. The presence of cocaine on toxicology reports was not statistically significantly associated with PPPD.
Conclusions
The authors conclude that the study findings suggest the inappropriate use of pregabalin among those who are known to misuse opioids and those in receipt of treatment for problematic drug use. More guidance and training for prescribers and treatment providers as well as the development of policies, including consideration given to scheduling pregabalin as a controlled drug, is recommended to better inform the public and medical practitioners of the potential harm due to ‘off label’ prescribing and of inappropriate use of pregabalin.
Close monitoring of prescribing practices, diversion, and misuse of pregabalin, especially among those who use opioids and within the treatment setting in Ireland, is urgently required. Any treatment with pregabalin should be subject to regular review with caution adhered to when considering prescribing pregabalin to women who are taking other drugs, especially CNS depressants. In Ireland, the nationwide implementation of an ePrescription system would assist in this process. In addition, an ePrescription system would help prevent people altering prescriptions or receiving multiple private prescriptions from different medical practitioners.
Table 1: Factors significantly associated with PPPD, 2013–2016 (n=1489)
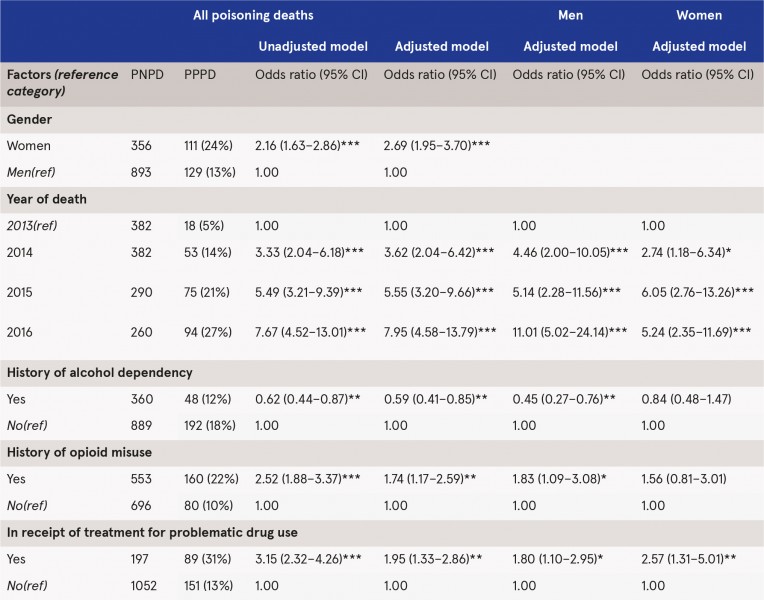
Adjusted model is for all predictors identified as significant in the unadjusted models. Variables significant at *** p<0.001; ** p<0.01; * p<0.05.
1 European Medicines Agency (2019) Pregabalin: summary of product characteristics. Amsterdam: European Medicines Agency. Available online at: http://web.archive.org/web/20190613071438/https://www.ema.europa.eu/en/documents/product-information/lyrica-epar-product-information_en.pdf
2 Lynn E (2019) Has an increase in the dispensing of pregabalin influenced poisoning deaths in Ireland? Drugnet Ireland, 71: 15–17. https://www.drugsandalcohol.ie/31455/
3 Lyndon A, Audrey S, Wells C, Burnell ES, Ingle S, Hill R, et al. (2017) Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction, 112(9): 1580–89.
4 United Nations Office on Drugs and Crime (2019) World drug report 2019. Booklet 3: Depressants. Vienna: United Nations Office on Drugs and Crime. https://www.drugsandalcohol.ie/30717/
5 McNamara S, Stokes S, Kilduff R and Shine A (2015) Pregabalin abuse amongst opioid substitution treatment patients. Ir Med J, 108(10): 309–310. https://www.drugsandalcohol.ie/24965/
6 Farmer D (2013) The prescribing and management of gabapentin and pregabalin in HM Prisons and Immigration Removal Centres in England: Collaborative audit report. NHS East & South East England Specialist Pharmacy Services. Available online at: https://www.sps.nhs.uk/wp-content/uploads/2013/07/Gabapentin20and20Pregabalin20Offender20Health20Audit20Report.pdf
7 Lynn E, Cousins G, Lyons S and Bennett K (2020) A repeated cross-sectional study of factors associated with pregabalin-positive poisoning deaths in Ireland. Drug Alcohol Depend, 206: 107741. https://www.drugsandalcohol.ie/31323/
Repository Staff Only: item control page