Guiney, Ciara (2025) Overprescribing of benzodiazepines, Z-drugs and gabapentinoids in Ireland. Drugnet Ireland, Issue 91, Spring 2025, pp. 17-21.
| Preview | Title | Contact |
|---|---|---|
|
PDF (Drugnet Ireland 91)
1MB |
The Examining the overprescribing of Benzodiazepines, z-drugs and Gabapentinoids in Ireland report was published by the Medical Council on 25 February 2025.1 The aim of the report was to outline key issues along with recommendations identified by the Overprescribing Working Group.
Working Group
The Overprescribing Working Group (‘the Working Group’) was established in 2019 by then President of the Medical Council, Dr Rita Doyle. Initially, the remit was to review concerns about overprescribing benzodiazepines and associated complaints received by the Medical Council. Z-drugs and gabapentinoids were added later. The Working Group consisted of representatives from a range of stakeholders, including the Health Service Executive (HSE); Department of Health; Pharmaceutical Society of Ireland (PSI); Irish College of General Practitioners (ICGP); Nursing and Midwifery Board of Ireland (NMBI); Health Products Regulatory Authority (HPRA); Irish College of Psychiatry; and Faculty of Pain Medicine, College of Anesthesiologists of Ireland.1
Medications
Benzodiazepines, Z-drugs, and gabapentinoids are mainly prescribed to reduce symptoms related to a range of psychological and neurological issues. They aim to enhance quality of life.1 The typical uses for each class of drug are as follows:
- Benzodiazepines (for example, diazepam and alprazolam) are sedatives that are used short term to manage anxiety, panic, addiction, alcohol detoxification, and insomnia.
- Z-drugs (for example, zopiclone and zolpidem) are used to treat insomnia.
- Gabapentinoids (for example, gabapentin and pregabalin) are used to treat pain.
The Misuse of Drugs Regulations 2017 provide controls for the prescribing of benzodiazepines and Z-drugs. Under this legislation, benzodiazepines are listed in Schedule 3 or Schedule 4 Part 1, whereas zolpidem and zopiclone are listed in Schedule 4 Part 1. Under Schedule 4 Part 1, the maximum length of a benzodiazepine or Z-drug prescription is 9 months.1 In Ireland, gabapentinoids are not classified as a controlled drug in the Misuse of Drugs Regulations 2017.1
Prescribing
Doctors are responsible for the safe prescribing of benzodiazepines, Z-drugs, and gabapentinoids. They adhere to drugs prescribing guidelines issued by the Medical Council.2 The Guide to Professional Conduct & Ethics for Registered Medical Practitioners guidelines document outlines the principles of professional practice that all doctors are required to follow.2 Registered nurses and midwife prescribers who have undertaken approved training must also adhere to legislation; professional regulation; and policies, procedures, and guidelines.1
Tracking use
Public health system
Benzodiazepine, Z-drug, and gabapentinoid usage is tracked using the Primary Care Reimbursement Service (PCRS). The PCRS processes payments to practitioners providing free or reduced cost services, using the General Medical Service (GMS) scheme.1 Pharmacies submit reimbursement claim data to the PCRS. This provides GMS-contracted doctors with an outline of their prescribing habits and how these habits relate to their peers. In addition, the PCRS operates a suite of Community Drug Schemes (CDSs); the GMS; the Drugs Payment Scheme (DPS); and the Long-Term Illness (LTI) Scheme. Drugs prescribed under these schemes are reported monthly and annually. Open data for all GMS drug claims (benzodiazepines and Z-drugs) can be found on the PCRS website.3
Only GMS data are used in Irish prescribing data analysis, which has been shown to represent only one-third of the population.4 However, 2018 figures captured under the PCRS Community Drug Scheme indicated that over 65% of the population had either a medical card, DPS eligibility, or LTI Scheme eligibility.1 The report further acknowledges that clinically active doctors (43%) in Ireland provide services publicly and privately; of this cohort, 6.8% only provide privately funded services.1 GMS data only account for doctors with a GMS contract who are providing publicly funded services.1
Open data are published for GMS benzodiazepine and Z-drug claims by community pharmacists monthly. Figure 1 shows that since 2018, public prescribing of benzodiazepines and Z-drugs has decreased each year. Figure 2 provides a breakdown of total monthly prescriptions between September 2021 and September 2023. The number of prescriptions for diazepam and alprazolam decreased between 2018 and 2023.
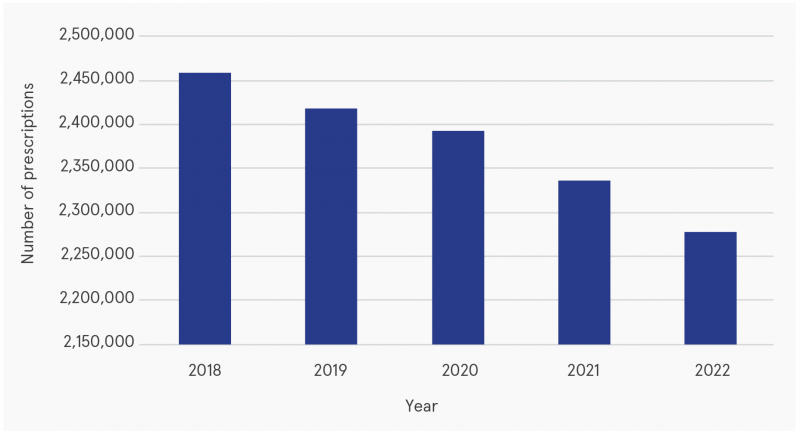
Figure 1: Number of prescriptions dispensed under the GMS for benzodiazepines and Z-drugs, 2018–2022
Source: Multiagency Working Group on Overprescribing (2025)1
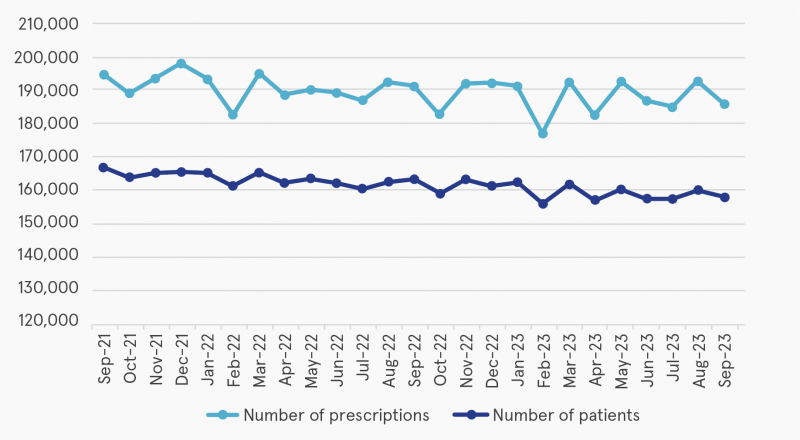
Figure 2: Total prescriptions and patients for benzodiazepines or Z-drugs between September 2021 and September 2023
Source: Multiagency Working Group on Overprescribing (2025)1
Outside public health system
No data are collected for non-GMS private prescriptions. This has been identified as an area of concern by the Working Group, who believe that legislative change will be needed in order to overcome this problem. Therefore, it is not possible to determine how much overprescribing occurs. Currently, getting access to a doctor’s private prescribing history is challenging in the event of prescribing complaints.1 It involves piecemeal trawling of prescriptions and contacting several individual pharmacies for records if patients have prescriptions dispensed in more than one pharmacy. The report acknowledges that this process is time-consuming, expensive, and inadequate with regard to patient safety and public protection.1 Moreover, no dispensing data are collected for patients in secondary care.1
Tracking internationally
Within the European Union, prescribing data are tracked by different countries in different ways.1 For example, in the Netherlands, there are three sources (general pracitioner (GP) electronic health records, community pharmacy databases, and insurer claims data). However, data linkage is not available.5 In contrast, prescription databases in Denmark, Finland, Iceland, Norway, and Sweden represent the overall population and contain data that could potentially be linked.1 Nordic countries have also started to implement processes to monitor medication use. The authors believe that the steps taken to establish these databases might provide important insights that could be considered within the Irish context (p. 27).1 In the United Kingdom, prescription data are stored centrally in the English Prescribing Dataset. This dataset does not include prescriptions prescribed and dispensed in prisons, hospitals, or privately; neither does it include prescriptions issued outside of England (in other words, in Scotland, Wales, or Northern Ireland). The National Health Service (NHS) also has a data warehouse called ePACT2. Authorised users are given access to these data in order to analyse them.
Specific issues in patient safety
Not having a centralised record of all prescriptions is deemed a risk to patient safety.1 However, due to amendments to the Medicinal Products (Prescription and Control of Supply) Regulations 2003 (as amended), prescriptions could be sent to patients’ chosen pharmacy electronically via Healthmail. Nonetheless, paper prescriptions are still used. The Working Group recognised that:
- Inappropriate use of benzodiazepines can result in addiction, adverse reactions, and poorer quality of life.
- Misuse of Z-drugs can result in sedative effects that may persist the next day.
- Higher doses of gabapentinoids are often used by those dependent on opioids to achieve a faster high and lower withdrawal.1
Complaints
The Medical Council’s Preliminary Proceedings Committee (PPC) is responsible for investigating complaints that arise regarding a doctor’s fitness to issue controlled drug prescriptions.1 Complaints relate not only to overprescribing but also to other prescribing issues. Between January 2018 and December 2022, 201 complaints were made in relation to the professionality of the prescribing doctor. Of these 201 complaints, 44 were referred for further investigation by the Fitness to Practise Committee. A further analysis of these complaints indicated that 32 complaints were in relation to overprescribing only. Of these 32 complaints, 23 (71.9%) were in relation to benzodiazepines, Z-drugs, and/or gabapentinoids.1 Complaints made regarding a doctor’s prescribing practice in relation to overprescribing fall into a ‘most serious category' (p. 38).1 When this happens, the Medical Council may take immediate action, which can result in the suspension of the doctor’s registration in Ireland until the complaint is concluded.1 Between January 2018 and December 2022, immediate action was taken against 19 doctors.
Patient supports
In September 2019, the Medical Council advised doctors to reduce overprescribing of benzodiazepines, Z-drugs, and gabapentinoids (pregabalin). However, it was acknowledged that this was challenging due to patients’ demands and having no access to counselling and services. The Working Group believe that the focus of this guidance is on GPs’ practice. However, GP prescribing has its limits because there is no system in place to report hospital-initiated prescriptions. The rationale for the advice was to move the focus towards patient safety.1 Moreover, public health campaigns outlining the risks of these benzodiazepines, Z-drugs, and gabapentinoids and their long-term addictive potential are lacking. The Working Group argue that having these campaigns would help doctors manage the dialogue with their patients.1 Further education and training for GPs, and more access to counselling and addiction services, would allow GPs to further support their patients.
Conclusion and recommendations
The Working Group have examined the issues of patient safety in relation to overprescribing and inappropriate prescribing of benzodiazepines, Z-drugs, and gabapentinoids. In light of their work, they encourage a more comprehensive understanding of patient safety issues. In addition, while they acknowledge the importance of existing data and the ability to identify trends, they identify the need to get access to all prescribing data. Finally, although a decrease has been evident in prescriptions for benzodiazepines and Z-drugs, the Working Group recognise the impact of overprescribing these drugs and how it affects patient safety.1
Drawing on evidence-based practice, the Working Group put forward several recommendations that aim to reduce dependence on these drugs. The main focus is on improved service delivery, education, and advancing transparency in prescribing practices. Moreover, consideration should be given to including pregabalin and gabapentin in the Controlled Drugs List. The Working Group believe that the effective implementation of the recommendations will need input from agencies across the Irish healthcare system. To achieve this, an implementation group should be established to evaluate ongoing progress.
1 Multiagency Working Group on Overprescribing (2025) Examining the overprescribing of benzodiazepines, z drugs and gabapentinoids in Ireland. Dublin: Medical Council. Available from: https://www.drugsandalcohol.ie/42740/
2 Medical Council of Ireland (2024) Guide to Professional Conduct & Ethics for Registered Medical Practitioners (9th edition). Available from: https://www.drugsandalcohol.ie/43275/
3 Health Service Executive (nd) Annual reports. Available from: https://www.sspcrs.ie/portal/annual-reporting/report/annual
4 Cousins G and Keenan E (2024) Gabapentinoids in Ireland (2010-2020): trends in prescribing, law enforcement drug seizures & post-mortem toxicology. Policy Brief, February 2024. https://www.lisbonaddictions.eu
5 Barbazza E, Verheij RA, Ramerman L, Klazinga N and Kringos D (2022) Optimising the secondary use of primary care prescribing data to improve quality of care: a qualitative analysis. BMJ Open, 12(7): e062349. Available from: https://www.drugsandalcohol.ie/43350/
B Substances > New (novel) psychoactive substances > Other novel substances > Gabapentinoids GABA (Pregabalin / Gabapentin)
B Substances > New (novel) psychoactive substances > Other novel substances > Zopiclone, eszopiclone, zaleplon and zolpidem
E Concepts in biomedical areas > Medical substance > Prescription drug (medicine / medication)
J Health care, prevention, harm reduction and treatment > Patient / client care management
T Demographic characteristics > Doctor / physician
VA Geographic area > Europe > Ireland
Repository Staff Only: item control page