Dillon, Lucy (2019) Medical use of cannabis and cannabinoids: Q&A for policymaking. Drugnet Ireland, Issue 69, Spring 2019, pp. 6-8.
| Preview | Title | Contact |
|---|---|---|
|
PDF (Drugnet Ireland 69)
1MB |
The medical use of cannabis and cannabinoids is a complex and challenging field. There is a wide range of issues to be considered by policymakers and other stakeholders when making decisions about the best approach to take. In December 2018, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) published a report on the topic to help support this process – Medical use of cannabis and cannabinoids: questions and answers for policymaking.1 It is supported by a background paper: A summary of reviews of evidence on the efficacy and safety of medical use of cannabis and cannabinoids.2
The report focuses on four main areas in relation to the medical use of cannabis and cannabinoids: a review of the evidence for their medical use across a number of conditions; the relevant regulatory frameworks; the approaches countries have used to allow access to them; and the regulatory challenges faced. This is a detailed report and just a selection of the key findings is presented below.
In what is a very fast-moving field characterised by an often hotly contested debate, this report has sought to provide an objective look at current evidence, practice and experience. (p. 34)1
Which substances are being discussed?
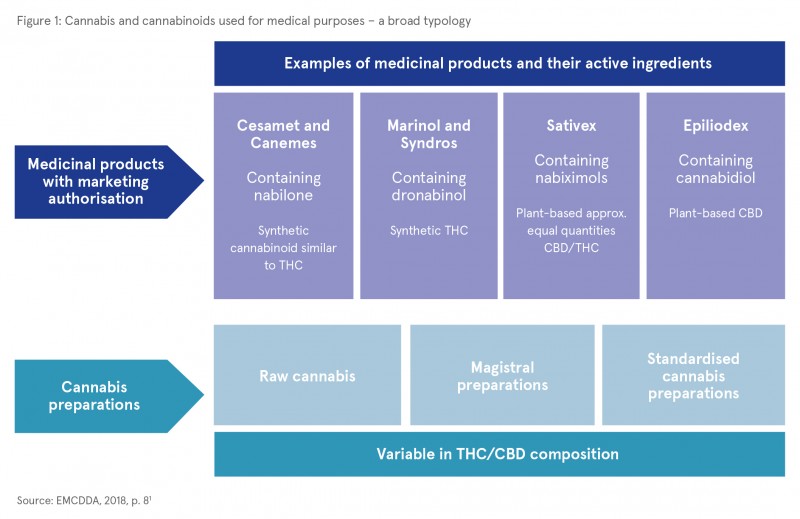
Broad terms such as ‘medicinal cannabis’ are often used when discussing this topic. The report highlights the need to define in a clearer way the kinds of substances involved. A broad typology is presented which makes a distinction between medicinal products that have a marketing authorisation (i.e. they have been authorised by a regulatory body as a medicinal product) and other cannabis preparations that have not been authorised for medical use (see Figure 1).
What evidence is there that cannabis and cannabinoids have medical uses?
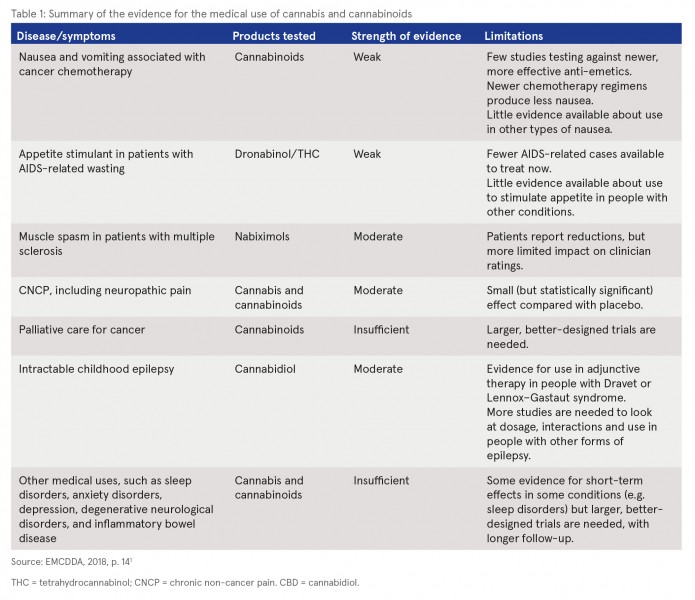
A summary of the evidence on the medicinal properties of cannabis and cannabinoids from systematic reviews of randomised controlled clinical trials is presented in the first part of the report.1,2 The report notes that interest in potential medical uses was only revived in the 1990s following the discovery of a cannabinoid system in the brain. This discovery suggested that cannabinoids could be used to treat chronic pain and neurological disorders such as multiple sclerosis and epilepsy. Therefore, it is acknowledged that there are gaps in the current evidence base and large well-conducted studies are scarce. See Table 1 for a summary of the key findings of the review of systematic reviews.
What approaches have countries used?
Overall, the report highlights the variation in approaches taken to allow access to cannabis and cannabinoids for medicinal use both within Europe and globally. They varied not only in terms of the actual products or preparations allowed, but also the regulatory frameworks adopted to control their provision. Three broad types of approach were identified, with countries often using more than one in parallel.
1 Allowing the use of medicinal products containing cannabinoids: Some countries have given market authorisation to medicinal products, thereby making them available for prescription.
2 Allowing the medical use of unauthorised products or preparations: Special access schemes have been established in some countries to allow for the medical use of unauthorised products or preparations. For example, it has been done as an interim measure while awaiting the findings of a clinical trial for a product or its authorisation. Others are made available on compassionate grounds and on a case-by-case basis. Physicians’ reluctance to prescribe for ethical and medico-legal reasons has presented major challenges to this approach.
3 De novo stand-alone medical cannabis programmes: Some countries have set up programmes that are outside the medicines regulatory systems. For example, in the United States the regulatory requirements for medicines were avoided by passing referendums allowing access to cannabis for broadly defined medical reasons. The authors note that this approach does not facilitate the conduct of clinical trials or establish an evidence base on which to assess the impact of these programmes.
What are the regulatory challenges?
The final part of the report highlights some of the regulatory challenges in allowing the medical use of cannabis and cannabinoids – in this context the authors are not talking about medicinal products rather cannabis preparations. They identify a number of questions, including:
- What types of products or preparations should be allowed?
- What forms should be allowed? For example, raw cannabis, magistral preparations made by a pharmacist, or others such as standardised cannabis extracts or oils?
- What routes of administration will be permitted? For example, oral or vaporised forms?
- For which medical conditions should treatment be available? Governments could limit access to those experiencing medical conditions for which there is evidence of efficacy, or take a broader approach where approval would be given for any conditions which some patients have reported benefits.
- Who would be allowed to prescribe the products? Would this be limited to a specialist physician or would any medical practitioner be able to do so?
- How would the government deal with any possible reluctance of practitioners to prescribe cannabis for any ethical or medico-legal reasons?
- Who will pay for the products or preparations? The patients, national healthcare system or health insurance schemes?
- What types of quality standards should be applied to the products? How would they be monitored and enforced?
- How will the necessary pharmacovigilance schemes and data collection for the International Narcotics Control Board be organised?
Concluding comment
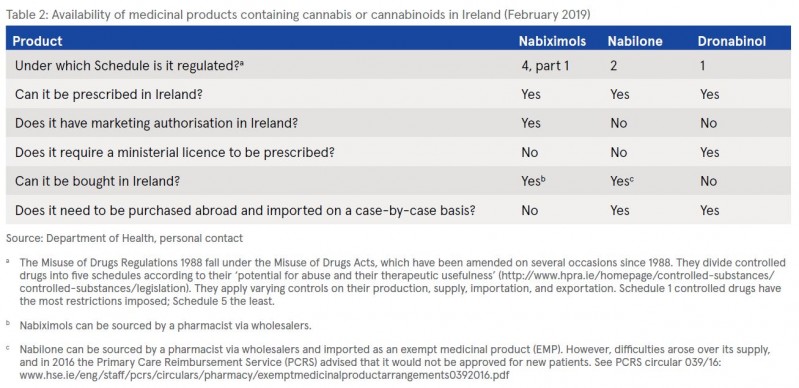
As with other EU countries, this is a topic that has attracted a lot of attention in Ireland and which is currently evolving under the work of the Expert Reference Group to advise on the development of a Medical Cannabis Access Programme.3 While Ireland is not specifically mentioned in the report when it discusses the approaches currently being used, the findings suggest a fit with a combination of allowing the use of medicinal products containing cannabinoids and allowing the use of unauthorised products or preparations (see Table 2 and Box 1).

1 European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2018) Medical use of cannabis and cannabinoids: questions and answers for policymaking. Luxembourg: Publications Office of the European Union. https://www.drugsandalcohol.ie/30034/
2 Hall W (2018) A summary of reviews of evidence on the efficacy and safety of medical use of cannabis and cannabinoids. Lisbon: European Monitoring Centre for Drugs and Drug Addiction. https://www.drugsandalcohol.ie/30208/
3 For further information, visit: https://health.gov.ie/cannabis-for-medical-use/
4 See Dáil Éireann debates: https://www.oireachtas.ie/en/debates/question/2018-11-29/164/
B Substances > Cannabis product (Cannabinoids)
E Concepts in biomedical areas > Medical substance > Medical / medicinal cannabis
G Health and disease > State of health
L Social psychology and related concepts > Legal availability or accessibility
MP-MR Policy, planning, economics, work and social services > Policy > Policy on substance use
MP-MR Policy, planning, economics, work and social services > Policy > Policy on substance use > Drug decriminalisation or legalisation policy
VA Geographic area > Europe > Ireland
Repository Staff Only: item control page