Dillon, Lucy (2024) Drug policy evaluation in Europe. Drugnet Ireland, Issue 87, Winter 2024, pp. 6-8.
| Preview | Title | Contact |
|---|---|---|
|
PDF (Drugnet Ireland 87)
2MB |
Since the early 2000s, the evaluation of drug policies and strategies has increasingly featured as part of the drugs landscape at European Union (EU) and member state levels. Evaluation is a critical part of the policymaking process. It supports effective policymaking by helping it adapt to changes over time, ensure lessons are learnt from previous successes and failures, and therefore strengthens value for money in the approach taken by the EU and member states to dealing with the drugs issue.
The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) considers policy evaluation a priority (see Box 1). In December 2023, it updated its online content for the topic overview on drug policy evaluation in Europe.1 Stakeholders can find links to sources to support policy evaluation, as well as examples of EU and member state outputs on the topic. The overview presents the state of play at the end of 2023 on the focus of national drug strategies and their evaluations.
Focus of national drug strategies
At the end of 2023, all 29 reporting countries (EU-27, plus Türkiye and Norway) had an active drug strategy. This compares to the mid-1990s when only one-third of the countries reporting to the EMCDDA had one, increasing to two-thirds in 2000. While some member states have a strategy that focuses specifically on illicit drug use, others (like Ireland) have a broader focus that covers other substances (e.g. alcohol, tobacco, and medicines) or addictive behaviours (e.g. gambling). Based on data collected through the 2023 national reports to the EMCDDA, Figure 1 illustrates the situation at the end of 2023 in terms of focus.
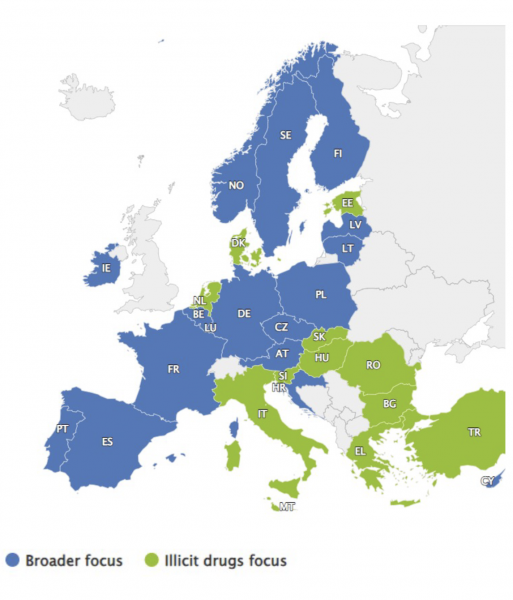
Source: EMCDDA (2023)1
Figure 1: Focus of national drug strategy documents reported by end 2023
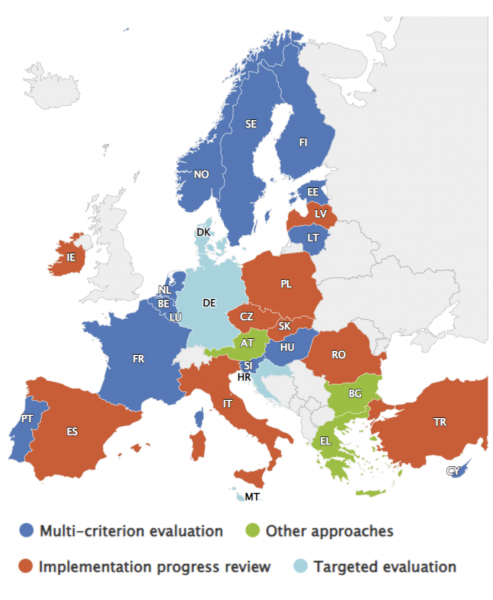
Source: EMCDDA (2023)1
Figure 2: National evaluations reported to the EMCDDA by end 2023
Evaluations of national drug strategies
Since 2010, the evaluation of national drug strategy documents is described by the EMCDDA as having become ‘standard practice’. Evaluations take many different forms depending on the focus, needs, and resources of national stakeholders, among other factors. To monitor evaluation practices across the member states, the EMCDDA has developed a typology that incorporates both whole-strategy and targeted evaluation, alongside ongoing monitoring and research aimed at supporting evaluation. These are:
- Implementation progress review: A review of the actions taken and/or the strategy’s context at its midpoint or end point.
- Multi-criterion evaluation: An evaluation of a strategy and/or action plan at its midpoint or end point.
- Targeted evaluation: An evaluation or audit of a specific policy or strategy aspect or area.
- Other approaches: Assessment by means of ongoing indicator monitoring, research projects, or regional or local strategy evaluation.
____________________________________________________
Box 1: Guide for commissioners and managers of policy evaluations
The EMCDDA’s seven-step guide to support the commissioning and managing of drug policy evaluations, published in 2017, continues to be a valuable resource. It offers an introduction to the topic and is designed to support those in the relevant roles in choosing the best approach to meet their needs, fit with their circumstances, and maximise the value of the evaluation. Four key messages were identified in the guide:
1 There is no one ‘correct’ way to perform an evaluation of drug policy. What is best will depend on what you want to know, what data you have available or can obtain, and the resources and time available to you.
2 Evaluation should not be seen as a one-off event but will be most useful if viewed as an ongoing process intertwined with policy or strategy development and implementation.
3 Evaluation needs to be accompanied by a commitment to taking action on the findings, and the opportunity to do so. The timing and choice of evaluation design need to be realistic and take this into account; producing a detailed evaluation of a previous strategy only after a new strategy has been developed and implementation begun will limit its usefulness.
4 Developing the expertise and data sources for drug policy evaluation over time will increase the ability to conduct evaluations in support of drug policy development and enhance action to address drug problems.
Source: EMCDDA (2017),2 p. 5
___________________________________________________
1 European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2023) Drug policy evaluation in Europe – topic overview. Lisbon: EMCDDA. Available from:
https://www.emcdda.europa.eu/publications/topic-overviews/policy-evaluation_en
2 European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2017) Evaluating drug policy: a seven-step guide to support the commissioning and managing of evaluations. Luxembourg: Publications Office of the European Union. Available from: https://www.drugsandalcohol.ie/27536/

Dr Sarah Morton, director of the Community Partnership Drugs Programme, University College Dublin, presenting at the National Drugs Forum 2023
Repository Staff Only: item control page